Antibiotic Elution
Pharmacokinetics of Gentamicin Eluted from a Regenerating Bone Graft Substitute - In Vitro and Clinical Release Studies
Authors: Stravinskas et al.
Publication: Bone & Joint Research (2016)
Summary: This study compares the elution of gentamicin from CERAMENT® G in vitro with elution and efficacy in clinical applications. The results showed that the elution pattern in vitro was comparable to that seen in patient studies.
Results:
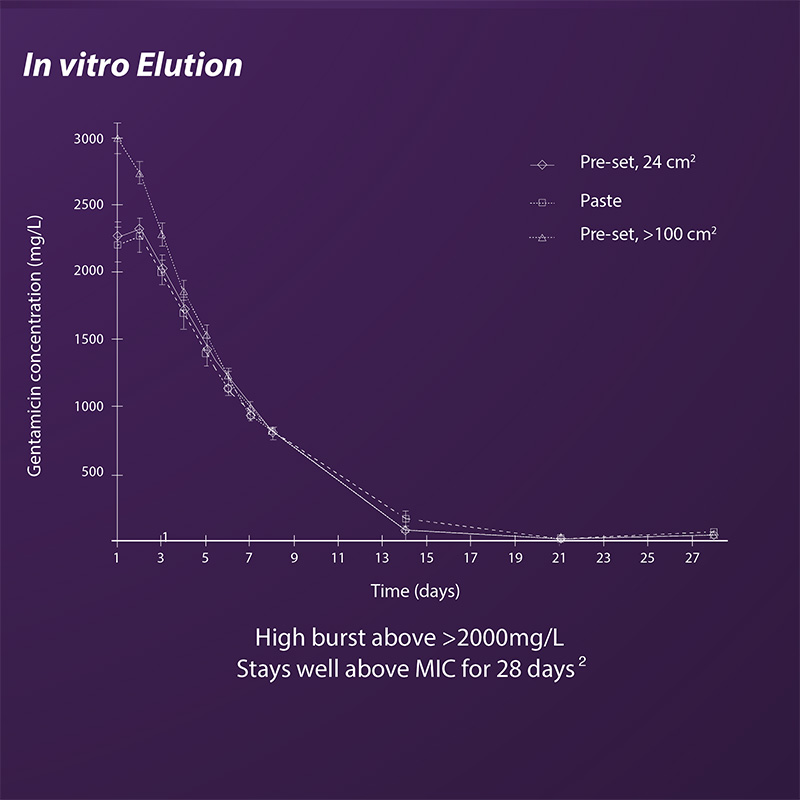
- Elution for up to 28 days
- Antibiotic elution is not surface dependent
- High initial burst of gentamicin above 2000 mg/L
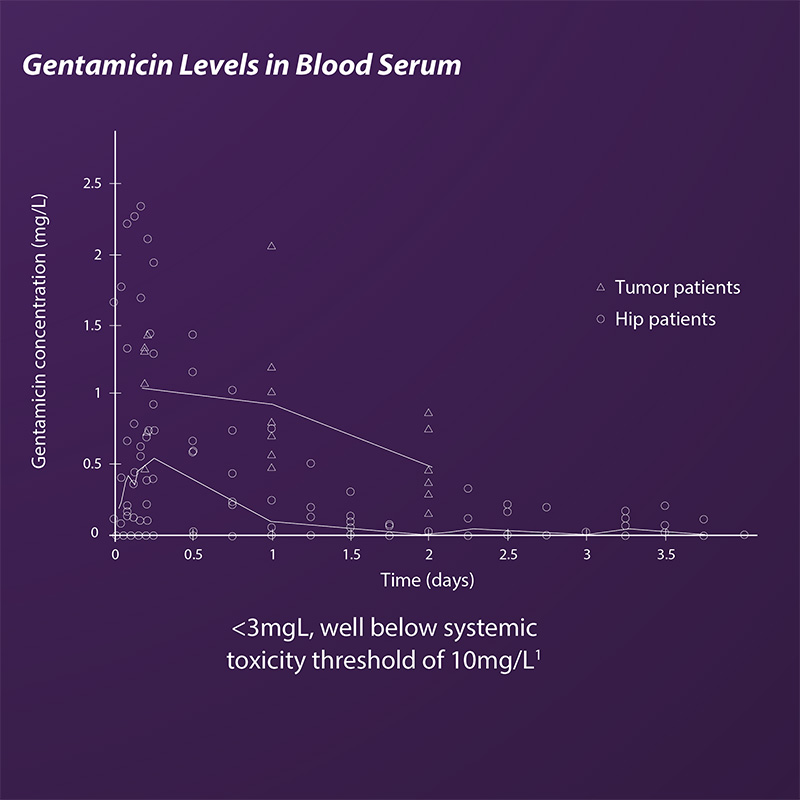
- Low serum concentrations well below toxic threshold of 10mg/L
Antibiotic Containing Bone Substitute in Major Hip Surgery: A Long Term Gentamicin Elution Study
Authors: Stravinskas et al.
Publication: Bone & Joint Research (2018)
Summary: Examines the antibiotic elution of CERAMENT® G in patients over a 30-day follow-up period. Also compares the pharmacokinetics of CERAMENT® G with gentamicin containing PMMA bone cement used in primary total hip arthroplasty.
Results:
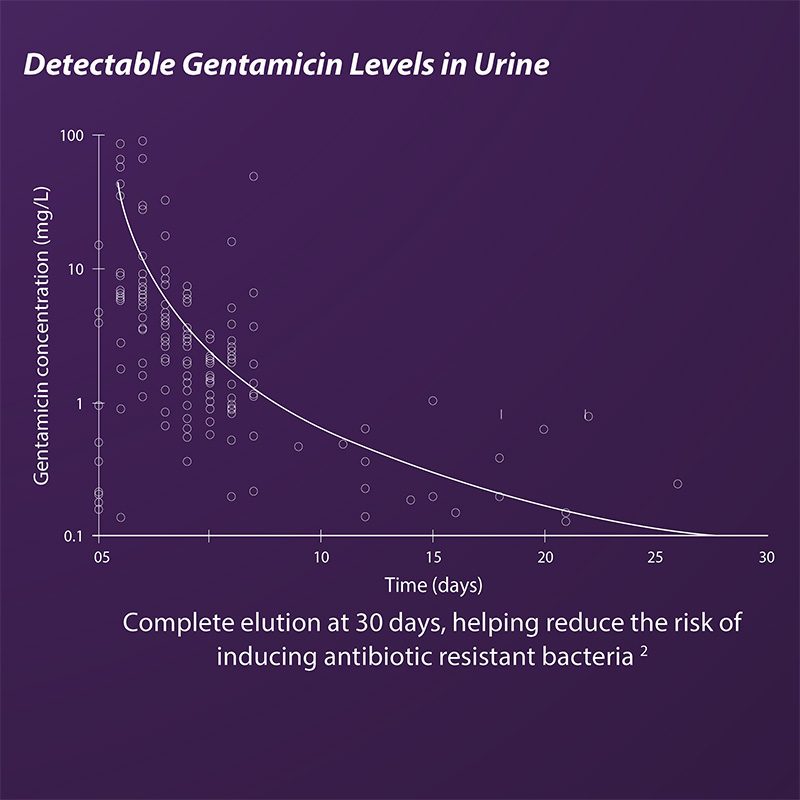
- All of CERAMENT’S antibiotic is released
- Antibiotic elution is not surface dependent
- CERAMENT® has higher initial elution than PMMA
- Low serum concentrations well below toxic threshold of 10mg/L