A New Pathway
Patient Friendly and Fiscally Prudent
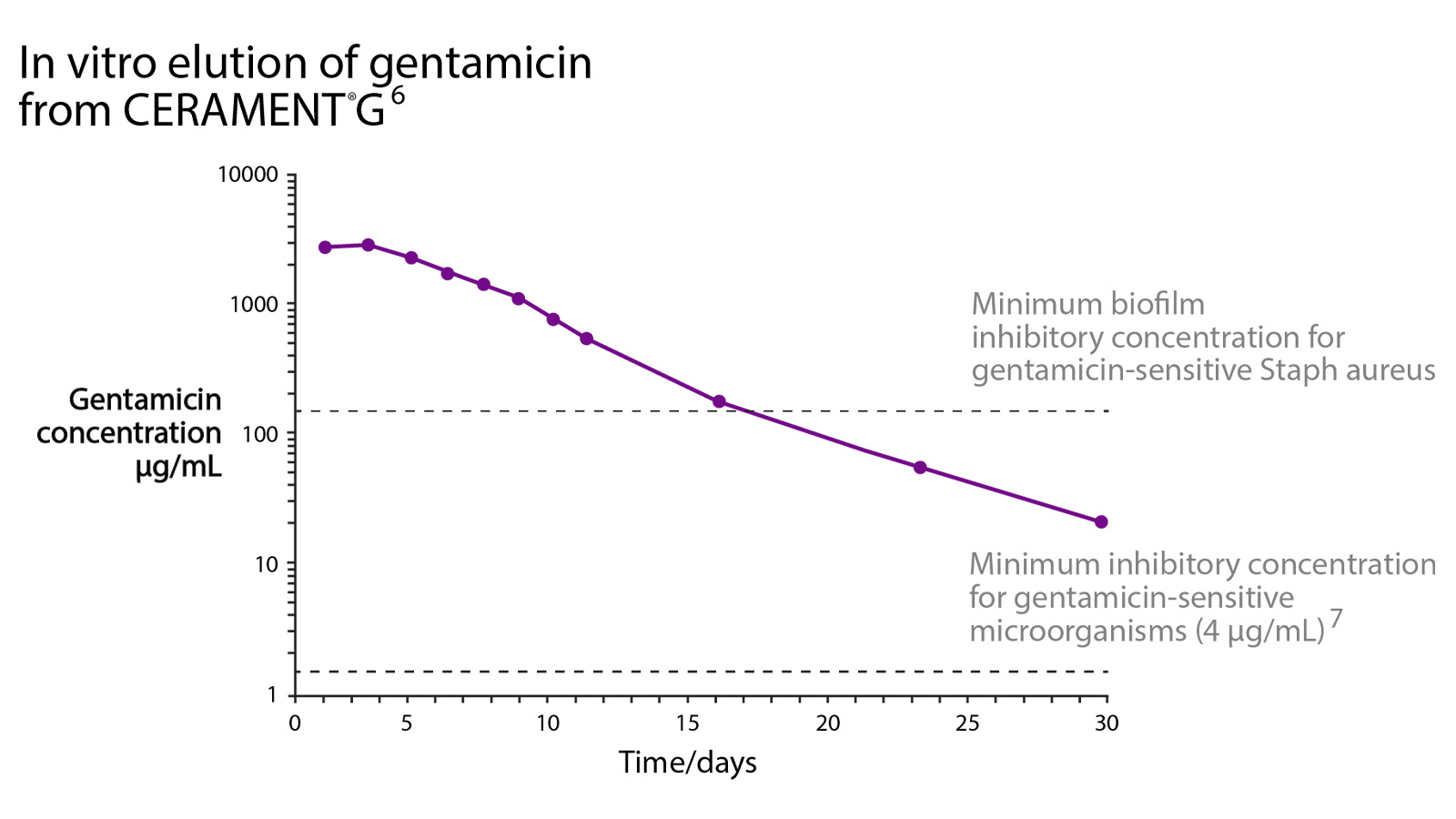
CERAMENT® G is uniquely designed to simultaneously provide bone remodeling and reliable, consistent and safe local antibiotic elution. Two clinical needs can thus be met in one single surgery which provides a pathway to significant cost savings and improved clinical outcomes.
An FDA-Designated Breakthrough Device
Therapies that treat serious conditions and have demonstrated substantial improvement or is the first of its kind.
CERAMENT® G
DUAL MODE OF ACTION
PROMOTE BONE REMODELING
• Proven bone remodeling4
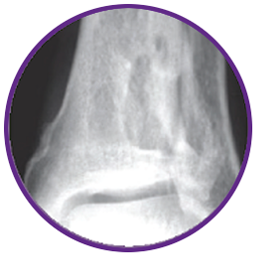
Debridement and Injection of CERAMENT® G:
CERAMENT® is highly flowable to completely fill voids and cracks.
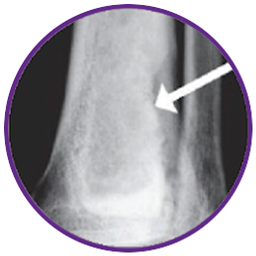
Bone remodeling within 12 months:
The calcium sulfate in CERAMENT® is fully resorbed, hydroxyapatite is embedded in bone and natural bone building continues increasing mechanical strength.4